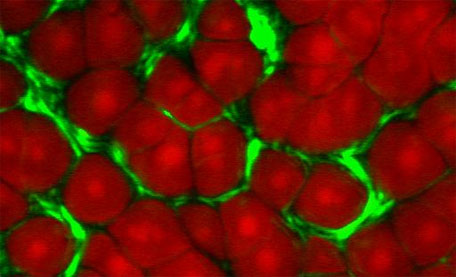
Multiphoton imaging and spectroscopy laboratory
Multiphoton imaging and spectroscopy
Multiphoton Microscopy (MPM) is a type of laser scanning microscopy that derives its contrast from non-linear optical properties of a sample and is currently attracting a great deal of interest across all fields of biophysics.
Non-linear imaging has many advantages over standard fluorescence confocal laser scanning microscopy, with the principle advantages being an increase in depth penetration and stain-free molecular contrast.
Contact

If you are interested in using the multiphoton laboratory or require further information please contact:
Dr Julian Moger
Senior Lecturer in Biological Physics
Tel: +44 (0)1392 724181
Fax: +44 (0)1392 724111
Email: J.Moger@exeter.ac.uk
The Multiphoton Imaging and Spectroscopy Laboratory is sponsored by Olympus UK Ltd.
Imaging mechanisms and equipment
Our facility combines three multiphoton contrast mechanisms in a single microscope, offering unrivalled flexibility allowing stain-free imaging of multiple species within a sample. As well as providing non-invasive image contrast, Multiphoton interactions also contain spectroscopic information which can provide novel mechanisms for detection and characterisation of biomolecules in-vivo.
Multiphoton Excitation (MPE)
MPE is the most simple form of Multiphoton Microscopy (MPM) which is used to excite fluorescence. A pulsed laser is used to provide simultaneous excitation with two (or three) photons of low energy, exciting the fluorophore to the same level as one high energy photon. MPE has many advantages over normal laser scanning microscopy.
The use of lower energy photons (infrared) reduces damage to the sample and also permits deeper penetration into scattering samples. Multiphoton excitation occurs only at the focal plane, removing the need for pinhole apertures.
As well as excitation of extraneous fluorophores, MPE can be used to excite indigenous tissue components such as NAD(P)H, flavins, and porphyrins.
Second Harmonic Generation (SHG)
SHG is a second-order nonlinear optical process, which requires an environment without a centre of symmetry to produce signals.
Two photons (ωp) are mixed in the sample to generate a third photon (2ωp). Several indigenous tissue components are known to exhibit SHG which can be used to generate label free images.
Collagen fibres are a well-known source of SHG. SHG deposits no energy into the sample due to the emitted SHG photon energy being equal to the total absorbed excitation photon energy.
Coherent Anti-Stokes Raman Scattering (CARS)
CARS is the most recent MPM imaging contrast. CARS microscopy derives contrast directly from Raman- active vibrational modes within molecules and requires two synchronised laser pulses of different wavelengths.
CARS is a third order nonlinear optical process in which a pump field Ep and a Stokes field Es interact with a sample to generate a signal field Eas at the anti-Stokes frequency of ωas = 2ωp - ωs. When ωp - ωs is tuned to be resonant with a molecular vibration (&Omega), the CARS signal can be significantly enhanced, producing a vibrational contrast.
Unlike spontaneous Raman scattering, CARS produces a highly directional field. The two excitation beams (ωp and ωs) form a beating field with frequency ωp - ωs. When ωp - ωs matches &Omega all the molecules within the interaction volume vibrate in-phase.
Advantages of CARS
CARS microscopy permits biological imaging with several advantages:
- Raman resonance enhancement provides chemical selectivity without the need for labelling.
- There is little scattering of the near-infrared excitation beams, allowing deep penetration in tissues.
- Due to the anti-Stokes shift, the CARS signal is of shorter wavelength than one-photon fluorescence. This allows detection in the presence of a strong fluorescent background.
- Coherent addition of CARS fields generates a large signal.
- Nonlinear dependence on excitation intensities produces inherent 3D resolution.
- Low absorption of the near-infrared excitation beams, significantly reduces the photodamage in biological samples.
Equipment
The front-end of our system is a modified Olympus IX71 inverted microscope. Dual sources, a pico-second OPO tunable from 700 – 1400nm (Levante Emerald from APE, Berlin), and a switchable femto- and pico-second system tunable from 700 – 1600nm (MIRA-D, and MIRA OPO from Coherent, USA) provide the multiple contrast modalities; Multiphoton fluorescence, Second- and Third-Harmonic Generation (SHG & THG) and Coherent Anti-stokes Raman Scattering (CARS).
Olympus FV300 and IX71 inverted microsope
- A modified Olympus FV300 and IX71 allows multiphoton imaging.
MIRA 900D
- Dual femto- and pico- second operation
- In femto mode provides excitation for second harmonic generation, multiphoton fluorescence, multiplex CARS and time-resolved CARS
- In pico mode provides excitation for frequency modulated CARS
- Output power: 1.2 W
- Wavelength Range: 690 - 990 nm
- Pulse width (femto mode): ~100 fs
- Pulse width (pico mode): ~1 ps
MIRA OPO
- Dual femto- and pico- second operation
- In femto mode provides excitation for third harmonic generation, sum frequency generation, pump-probe microscopy
- In pico mode provides excitation for frequency modulated CARS
- Output power: 300 mW
- Wavelength Range: 1050 - 1600 nm
- Pulse width (femto mode): ~120 fs
- Pulse width (pico mode): ~2 ps
Levante OPO
- This is a picosecond OPO pumped by a frequency doubled Nd:YVO-Laser (523 nm). The signal and idler are tuneable from 690 to 990 nm and 1150 to 2300 nm respectively.
- There is perfect spatial and temporal overlap of signal and idler beam, which can be outcoupled on one joint beam axis.
Adjacent Cell Culture Room
- Class II cell culture hood
- Incubator